Cow’s milk allergy (CMA) is associated with dysbiosis and increased susceptibility for infections, and it has been suggested that it can be managed (in part) by pro-, pre-, and synbiotics.1
With a growing body of research investigating the gut microbiota, immune responses and outcomes, it is important to understand the role of modulating an infant’s gut microbiota in the management of formula-fed cow’s milk allergic infants.
Early life is a critical time for the development of the gut microbiota and the maturation of the immune system. As the infant gut is almost sterile at birth, it must be colonised by different bacteria to form the gut microbiota. Similarly, the infant immune system is also naïve at birth and must learn to distinguish between threats and “friends”. Both these developmental processes work together.2
Immune System Development and the Gut Microbiota
With 70-80% of immune cells residing in the gut, microbial interactions are important drivers in the maturation of the immune system.3 The gut microbiota plays a pivotal functional roles within the body including defence against harmful pathogens, strengthening the body’s immune defences and performing vital metabolic tasks.4 For infants and children, it is especially important to maintain the balance between the gut microbiota and the immune system given its role within health.5
Why are Synbiotics Important in the Management of CMA?
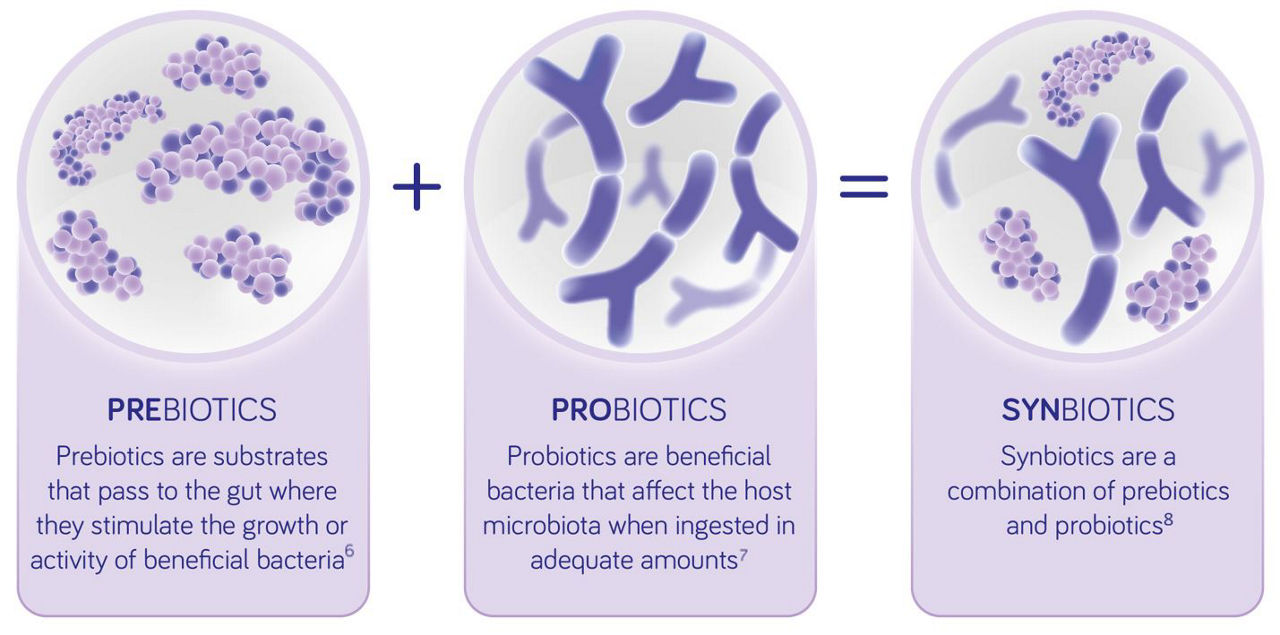
Synbiotics are a combination of pre- and probiotics.9 They can influence the immune system directly, or indirectly, via the gut microbiota, and therefore may play a role in influencing the course of allergic disease.10 The objective of combining pre- and probiotics is to achieve stronger positive effects than with either component alone so that they are working synergistically.11
The Impact of Gut Microbiota Dysbiosis on Health and the Development of Allergy
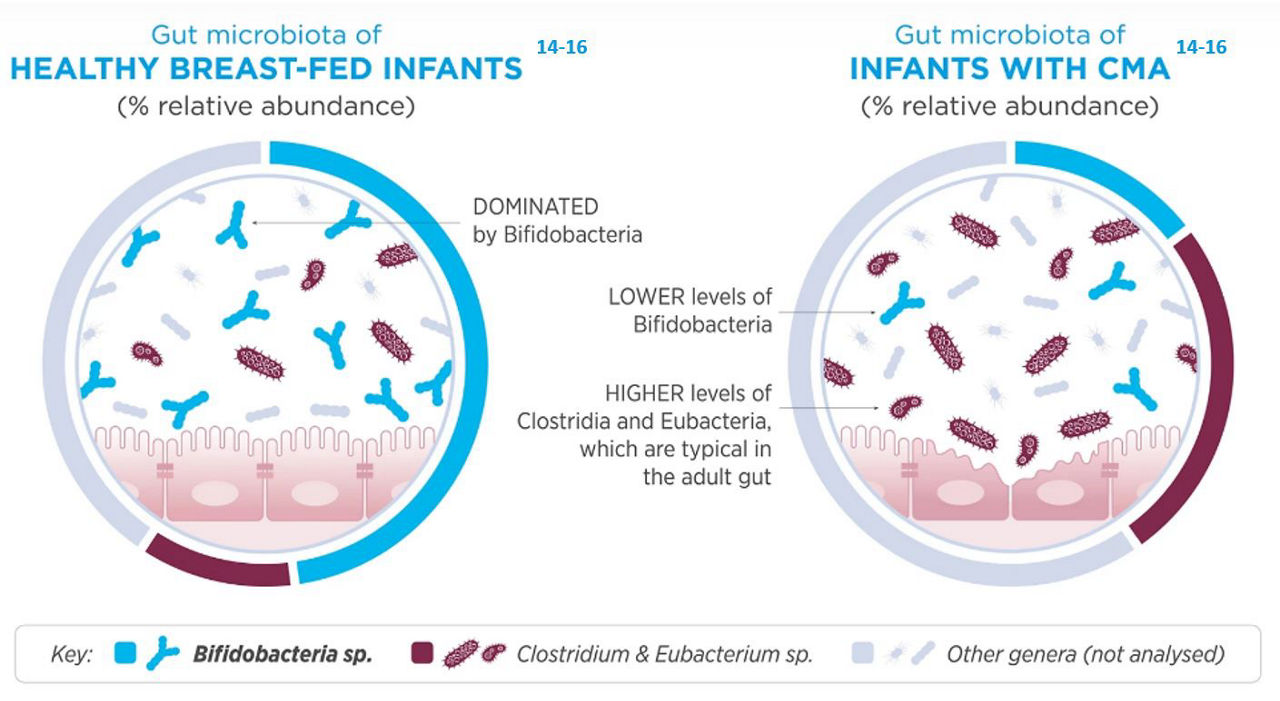
Healthy breast-fed infants typically have a Bifido-dominant gut microbiota, meaning that the gut has a higher amount of Bifidobacteria than other species. The Bifidobacteria species are transmitted via the birth canal and from the mother’s breast milk.12,13
In addition to bacteria, breast milk also contains non-digestible oligosaccharides that are readily consumed by these same species of Bifidobacteria. Cow’s milk allergic infants often have a different profile of gut microbes when compared to a healthy breastfed infant, this is characterised by much lower levels of Bifidobacteria and higher levels of adult like bacteria. This altered microcrobial composition is termed dysbiosis.14-16
Future of Allergy Management for CMA Patients
Consideration should be given to:
- The aberrant gut microbiota associated with allergy and the key role of the gut microbiota on immune system maturation
- The increasing clinical evidence for potential benefits of managing formula fed infants with CMA using hypoallergenic formulas supplemented with synbiotics
- Jensen et al. World Allergy Organization Journal (2022) 15:100668
- Knol et al. EMJ. 2020;5(4)20-28
- Vighi G et al. Clinical and Experimental Immunology.2008;153:3-6
- O’Hara A et al. EMBO Rep. 2006;7:688-93
- Andrew J et al. Immunology 2014;142:24-31
- Gibson GR, et al. Nat Rev Gastroenterol Hepatol 2017; 14(8): 491–502.
- Patel R, et al. Clin Perinatol 2013; 40(1): 11-25.
- Schrezenmeir J, et al. Am J Clin Nutr. 2001;73(Suppl 2):361S-364S.
- Gibson GR, et al. J Nutr, 1995 Jun;125(6):1401–1412.
- Agostoni C, et al. J Pediatr Gastroenterol Nutr, 2008;46(1):99110.
- Shamir R, et al. Gut Health in Early Life: Significance of the Gut Microbiota and Nutrition for Development and Future Health. 2015; John Wiley and Son.
- Jeurink, et al. 2012, Beneficial Microbes 4(1):17–30.
- Backhed F, et al. Cell Host Microbe 2015:17(5):690– 703.
- Canani RB, et al. ISME J. 2016;10(3):742–750.
- Thompson-Chagoyan OC, et al. Pediatr Allergy Immunol. 2010;21(2p2):e394–e400.
- Candy DCA, et al. Pediatr Res. 2018;83(3):677–686
Help us provide information most relevant to you
Please ensure your role and areas of interest are up to date.